Claim
Low voltage electrolysis raises seawater alkalinity in a closed system if the chlorine gas produced is adsorbed by activated carbon. Also, the seawater alkalinity increase happens within minutes and is greatest near the cathode.
Evidence
The alkalinity of Hanalei Bay is 7.0 dKH, the pH is 7.8, and there is no detectable chlorine in the water. Our first experiment was conducted in a 25-gallon system with an electrolysis voltage of 3.5 volts generating a 0.3 amp electrical current. That system had no flowmakers, so the water was not fully mixed. The anode and cathode were energized for 5 minutes, after which data was quickly collected from eight different points within the system. The average alkalinity of those samples was 6.7 dKH, significantly lower than the starting seawater value of 7.0 dKH.
This was surprising and very important data. Our original hypothesis was that electrolysis would increase alkalinity. After seeing the opposite result in our experiment, we formed a new hypothesis: the acidic chlorine compounds created by seawater electrolysis react with the buffering of carbonate ions and lower the alkalinity in the closed system.
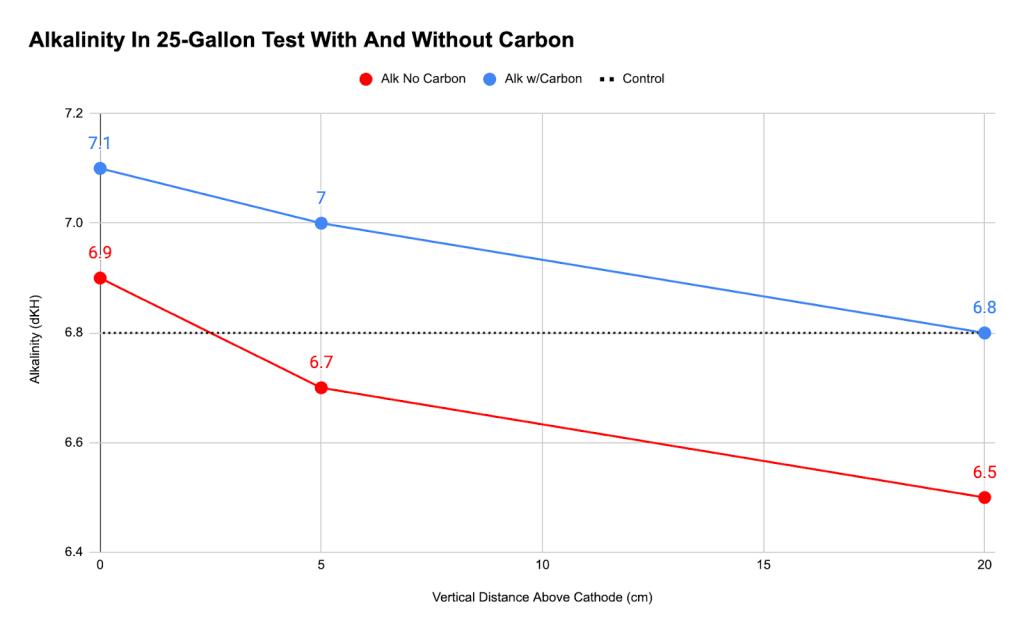
With that in mind, we covered the anode with mesh bags of activated carbon and repeated the experiment. We hoped that the carbon would absorb the chlorine. The results for that experiment are compared with the first experiment below, using measurements taken at the cathode and 5 cm and 20 cm above the cathode.
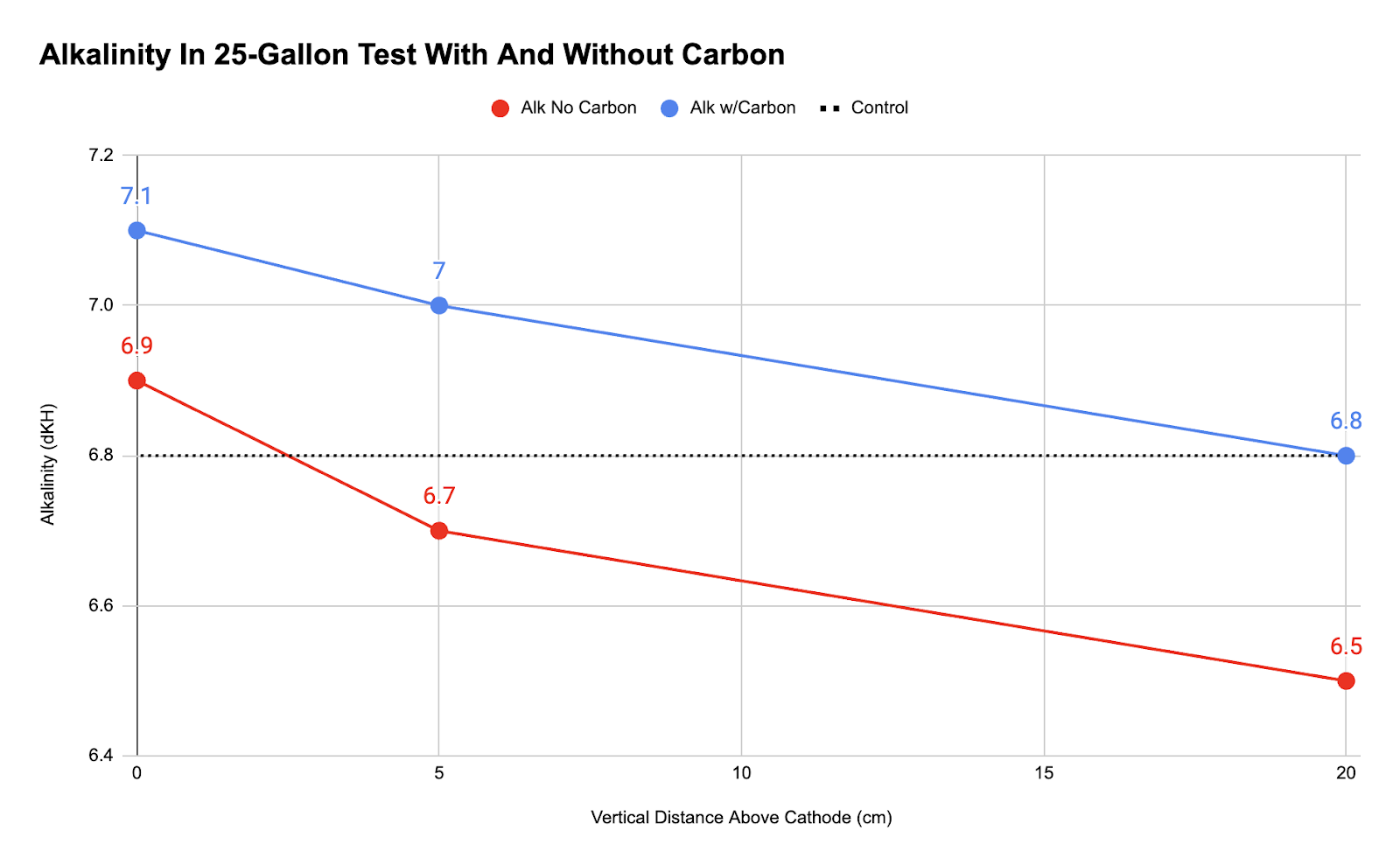
In the experiment without carbon, the alkalinity was lower. In the experiment with carbon, the alkalinity was higher. This shows that electrolysis raised alkalinity, but only if the chlorine is nullified. In addition, the slope of the lines shows that the the water near the cathode always had a higher alkalinity than the control and the farther you get away from the cathode, the lower the alkalinity gets. This proves that the cathode directly increased local alkalinity, and putting activated carbon over the anode adsorbs chlorine and reduces the production of hypochlorous acid, thus increasing alkalinity.
In addition, we sampled the pH at eight points in both experiments and in both cases, the average pH across the eight samples was 7.9 compared to the base seawater pH of 7.8. Electrolysis does not significantly affect pH regardless of the presence of activated carbon.
We did a final pair of “blitz” experiments at a much higher amperage to further back up our theory that chlorine reduced alkalinity. First, we ran 10 amps at 17 volts through a small 5-gallon system, and this time, we only waited 5 seconds before drawing water directly from the anode. That water had a chlorine concentration of 2.78 ppm and an alkalinity of 0, indicating that chlorine in the water causes a drastic decrease in alkalinity. In addition, that free chlorine concentration is approximately 300 times higher than the 0.009 ppm EPA level of concern for marine invertebrates, making life in the experimental tank impossible. So, the next step was to eliminate free chlorine.
Thus, after wrapping the anode in activated carbon, we ran a final “blitz” experiment with the same 5-gallon system at 10 amps and 17 volts. This time, the level of free chlorine was 0 ppm, indicating that the activated carbon was able to absorb all the chlorine produced by the electrolysis. We also took three alkalinity samples at zero seconds, 30 seconds, and 60 seconds, as shown below.
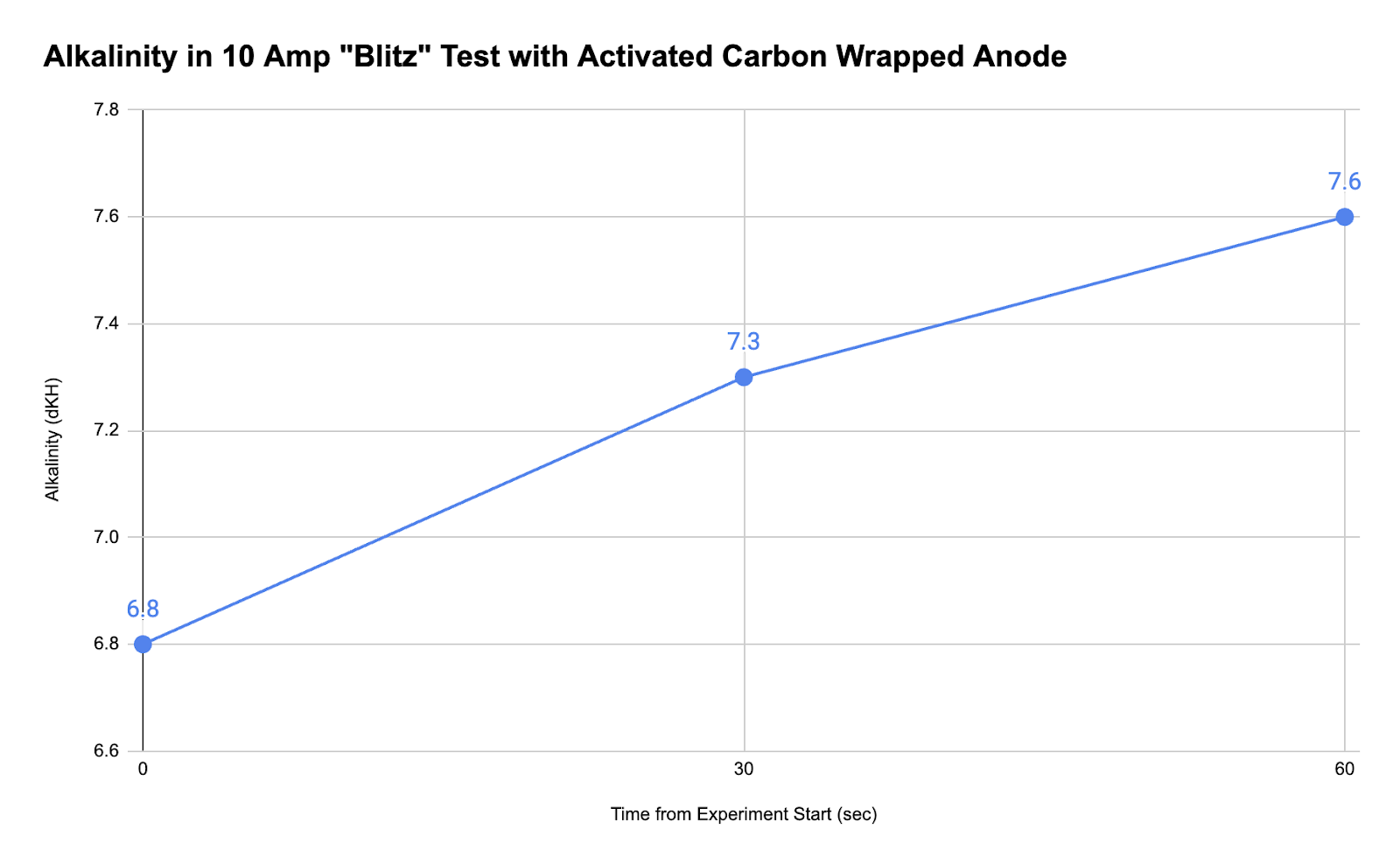
Wrapping the anode in carbon adsorbed the chlorine gas and reduced or prevented the production of hypochlorous acid, allowing alkalinity to rise rapidly after the start of electrolysis.
Conclusion and Next Steps
All this data proves our updated hypotheses: passing a current through seawater increases local alkalinity, free chlorine reduces alkalinity but it can be adsorbed by activated carbon, and alkalinity rises rapidly when electrolysis starts and is higher closer to the cathode.
Some experiments have shown that increased alkalinity increases coral growth. However, whether or not the increase in alkalinity is the only thing causing the coral to grow faster during electrolysis is unknown. This experiment showed us that the effects of electrolysis in a closed environment are drastically different than the results in the open ocean. However, it also showed us that activated carbon is a solution to the problem of free chlorine and hypochlorous acid. That solution allows us to take the next step in figuring out how to use electrolysis in a closed environment to study how electrolysis can be used to increase reef resiliency.
Leave a comment