Here is my research proposal for my first project! We completed the research in early June and I am working on the analysis now. I look forward to sharing more about how we performed the experiment, and, best of all, the results!
Electrolysis of Seawater: Effects of Low-Voltage on Alkalinity and Other Critical Parameters Required for Coral Reef Resiliency
Rationale
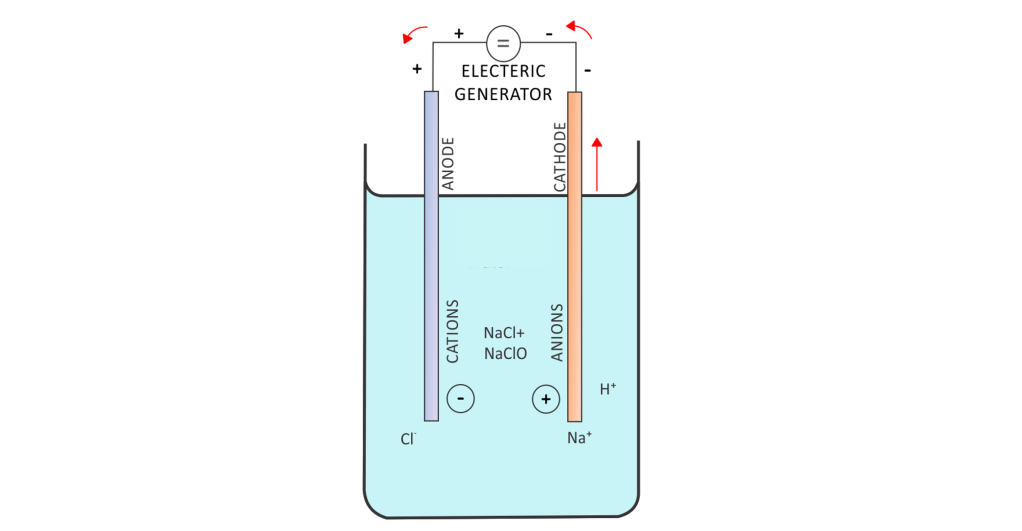
Passing an electric current through seawater results in electrolysis. The effects of electrolysis on seawater are myriad, but mainly driven by the electrolysis of water and salt. The H2O is separated into H+ and O2, the H+ forms H2 at the cathode. In addition, OH– is also formed near the cathode. That OH– raises the pH around the cathode. The NaCl present in seawater is also affected by electrolysis; it turns into gaseous Cl2 and Na+. The Cl2 forms at the anode, and the Na+ ions remain in the water. The Cl2 quickly reacts with H2O to form HClO hypochlorous acid. CaCO3 accretes on the cathode during electrolysis, as its water solubility decreases with higher pH and the water near the cathode is very basic due to the presence of OH– ions. The CaCO3 dissolves in water, forming free-floating Ca2+ atoms and CO32- molecules. In normal seawater, those CO3 molecules undergo a buffering reaction to form either Carbonic acid( H2CO3), CO2, or H2O. The positive Ca2+ atoms naturally migrate towards the cathode and combine with the OH– to form Ca(CO3)2, or calcium bicarbonate. That calcium bicarbonate raises the alkalinity of the water near the cathode.
Corals require alkalinity for skeletal growth, and some research has shown that higher alkalinities benefit coral growth and health. In particular, high alkalinity drastically affects corals’ ability to recover from shock. That benefit will become increasingly important as global temperatures fluctuate more and more. In addition, high alkalinity reduces the impact of ocean acidification. Alkalinity impacts how many H+ and OH– ions are required to affect the pH by buffering seawater. Since recent ocean acidification is caused by the increase in the concentration of carbonic acid due to higher CO2, raising the local alkalinity of reefs has the ability to partially reduce the impacts of ocean acidification. It is unknown whether seawater electrolysis has an effect on the alkalinity of an entire reef or just the water near the cathode. Beginning to answer that question is the primary purpose of this research, with the goal of… In addition, this lab will help determine the issues that might arise from producing Cl2 gas. It will also show how electrolysis can affect magnesium and calcium concentrations in a reef. The true impacts of the previously mentioned pH buffering will also be revealed. Beginning to answer those questions will inform the design of an aquarium apparatus for investigating the effects of low-voltage electrolysis on live coral with the goal of understanding how low-voltage electrolysis can be used to support the adaptability, health, biodiversity and growth of critical reef ecosystems in the face of ocean acidification, eutrophication, heat stress and associated disease.
Questions & Hypotheses
Questions:
- What is the effect of seawater electrolysis on the larger alkalinity and pH of seawater in a contained environment?
- What is the effect of seawater electrolysis on the local alkalinity and pH of seawater at the cathode?
- How does the does the voltage used during electrolysis affect the larger alkalinity and pH in seawater?
- What is the effect of seawater electrolysis on the larger chlorine levels in a contained environment?
- How much faster does electrolyzed seawater evaporate than seawater that is not electrolyzed?
Hypotheses:
Seawater electrolysis will have a slight impact on the alkalinity of the whole container but will have a large impact on the water directly near the cathode.
A larger amount of volts used during electrolysis will result in a larger increase in alkalinity, but will also make the water evaporate faster.
Procedures
The first step is to test alk, pH, chlorine, and salinity of the Hanalei Bay.
The experiment will be run twice, once with the lowest voltage possible, and again at 2x that lowest voltage. A test will be done to determine the lowest possible voltage as it depends on the salinity of the seawater and how large and far apart the electrodes are.
5-gallon buckets will be used for the long-term tests, as the total alkalinity changes would happen faster. For the short-term tests, a 30-gallon cooler will be used, as it allows measurements to be taken from farther away from the cathode/anode. The exact amount of seawater added will be measured to ensure there are no irregularities.
For the first short-term test, power will be sent to the anode and cathode at minimum voltage, and after 1 minute, samples will be taken at the cathode, 5cm and 20cm above the cathode, 5cm and 20cm horizontal from the cathode, and 5cm and 20cm diagonal from the cathode. pH and alkalinity will be tested as well. A test will also be taken exactly halfway between the cathode and anode.
Following that, the seawater will be replaced, and power will once again be turned on at minimum voltage with a pump mixing the water. The experiment will run for 6 hours, and once 6 hours have passed the testing protocols mentioned above will be used.
To answer the questions about evaporation and chlorine levels, two 5-gallon buckets will be set up in the shade. A line will be drawn at the water level of each bucket. One will be electrified at 2x the minimum voltage from the short-term experiments, the other will be the control. The test will take place for 2 days. Voltage, amperage, temperature, overall alk, pH, chlorine and salinity will be tested approximately every 12 hours. At the end of 48 hours, the distance from the line to the current water level will be measured, in addition to the weight of the bucket.
The pH and alkalinity data will be collected with a Hanna Instruments (HI 97115) multi-tester and double-checked with a Hanna (HI 772) DKh checker as needed. Total chlorine will be measured with a Hanna HI711 total chlorine checker. Salinity will be measured with an Agriculture Solutions Portable Refractometer.
Risks and Safety
Electrolyzing water results in chlorine gas, which is very dangerous to humans. It causes chemical burns and, if exposed in large enough quantity, can cause death. To address that, the test will happen outside and adequate ventilation will be present. We will also use the lowest current possible, below 1A, as chlorine gas production is directly related to total current.
- Chlorine gas generated is maximum 9 liters/day/amp or 0.006 liters/min/amp; the actual amount released is significantly less than this due to reactions in seawater: https://cdn.technologynetworks.com/ep/pdfs/electrolysis-halogen-oxidizing-agents-and-reef-restoration.pdf and https://www2.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/electrochemistry/07electrolysis/18_72.htm
- Chlorine concentration risks (24-hr 0.5ppm, 1-hr 3ppm, IDLH 30ppm): https://www.cdc.gov/niosh/idlh/7782505.html
- Chlorine MSDS: https://www.airgas.com/msds/001015.pdf
Electricity can easily cause electric shocks in the presence of seawater. All AC 120V equipment will be grounded in a GFCI circuit and protected from exposure to seawater following standard marine aquarium safety practices. The DC voltages used in the electrolysis cell will be kept below 10V, which is well below the 30V to 50V maximum threshold considered safe for humans.
- Low-voltage safety limit of 50v: https://wiki.classe.cornell.edu/Safety/Handbook/ElectricalSafety
- General aquarium safety procedures: https://www.bulkreefsupply.com/aquarium-safety
Data Analysis
The research questions all have simple answers. However, to properly answer them, many different things will be measured at once. The measurements to be taken are listed below.
| Alkalinity (dKH) | pH | Chlorine (ppm) | Salinity (SG) | Temp (C) | Ca (ppm) | Mg (ppm) | |
| Hanalei Bay |
Minimum Electrolysis Voltage _____
Short-term test #1 – No Flow, Minimum Voltage
Voltage _____
Current _____
| Alkalinity (dKH) | pH | Chlorine (ppm) | Salinity (SG) | |
| 0 cm (top of cathode) | ||||
| 5 cm vertical | ||||
| 20 cm vertical | ||||
| 5 cm horizontal | ||||
| 20 cm horizontal | ||||
| 5 cm at 45-degrees | ||||
| 20 cm at 45-degrees | ||||
| Between anode and cathode |
A graph will be drawn to show how the chemical levels vary with distance from the cathode.
Short- term test #2 – No Flow, Double Voltage
Voltage _____
Current _____
| Alkalinity (dKH) | pH | Chlorine (ppm) | Salinity (SG) | |
| 0 cm (top of cathode) | ||||
| 5 cm vertical | ||||
| 20 cm vertical | ||||
| 5 cm horizontal | ||||
| 20 cm horizontal | ||||
| 5 cm at 45-degrees | ||||
| 20 cm at 45-degrees | ||||
| Between anode and cathode at bottom of container |
A graph will be drawn to show how the chemical levels vary with distance from the cathode.
Short-term test #3 – No Flow, Double Voltage With Activated Carbon
Voltage _____
Current _____
| Alkalinity (dKH) | pH | Chlorine (ppm) | Salinity (SG) | |
| At cathode | ||||
| 5 cm vertical | ||||
| 20 cm vertical | ||||
| 5 cm horizontal | ||||
| 20 cm horizontal | ||||
| 5 cm at 45-degrees | ||||
| 20 cm at 45-degrees | ||||
| Between anode and cathode at bottom of container |
A graph will be drawn to show how the chemical levels vary with distance from the cathode.
A graph will be drawn to show how the chemical levels vary with distance from the cathode.
A chart will be made showing the differences in the measurements from the four tests.
Six-Hour Test – No Flow
| Alkalinity (dKH) | pH | Chlorine | Salinity (SG) | |
| Between anode and cathode | ||||
| 20 cm away diagonal |
Bibliography
Electrochemical CO2 capture and storage with hydrogen generation – Core. Accessed May 2023. https://core.ac.uk/download/pdf/81198605.pdf.
Electrolysis, halogen oxidizing agents and reef restoration – UC Santa Cruz, Koster. Accessed May 2023. https://cdn.technologynetworks.com/ep/pdfs/electrolysis-halogen-oxidizing-agents-and-reef-restoration.pdf and https://escholarship.org/content/qt1vb5b1j6/qt1vb5b1j6_noSplash_168ab17130033365c8c92a8151e1f410.pdf.
Electrically stimulated artificial mussel (mytilus edulis) reefs to create shoreline protection and coastal habitat in St. Margaret’s Bay, Nova Scotia – Dalhousie University, Miller. Accessed May 2023. https://dalspace.library.dal.ca/bitstream/handle/10222/80265/Miller_S_MMMGraduateProject.pdf?sequence=1&isAllowed=y.
Grow corals faster with elevated reef tank parameters? – BRStv. Accessed May 2023. https://www.bulkreefsupply.com/content/post/grow-corals-faster-with-elevated-reef-tank-parameters-brstv-investigates.
Reef tank pH, benefits of raising saltwater aquarium pH – BRStv. Accessed May 2023. https://www.bulkreefsupply.com/content/post/reef-tank-ph-benefits-of-raising-saltwater-aThresholds and drivers of coral calcification responses to climate changequarium-ph-brstv-investigates.
Could artificial ocean alkalinization protect tropical coral ecosystems from ocean acidification? – Environmental Research Letters, Feng et al. Accessed May 2023. https://iopscience.iop.org/article/10.1088/1748-9326/11/7/074008.
Ocean acidification – Smithonian. Accessed July 2023. https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification.
Electrical stimulation greatly increases settlement, growth, survival, and stress resistance of marine organisms – Natural Resources, Goreau. Accessed July 2023. https://www.scirp.org/pdf/NR_2014073118345172.pdf.
Coral reef electrotherapy: Field observations – Frontiers in Marine Science, Goreau. Accessed July 2023. https://www.frontiersin.org/articles/10.3389/fmars.2022.805113/full.
The effects of electrical voltage differences and initial fragment size on growth performance and survival rate of coral acropora cerealis in Biorock method – Journal of Aquaculture & Marine Biology, Natasasmita et al. Accessed July 2023. http://medcraveonline.com/JAMB/JAMB-04-00086.pdf.
Thresholds and drivers of coral calcification responses to climate change – Global Change Biology, Kornder et al. Accessed July 2023. https://onlinelibrary.wiley.com/doi/full/10.1111/gcb.14431.
Decreased growth of stylophora pistillata with nutrient-Driven Elevated Zooxanthellae Density is Largely Explained by Dissolved Inorganic Carbon [90% Carbonate] Limitation – Reefs.com, Hylkema et al. Accessed July 2023. https://reefs.com/magazine/decreased-growth-of-stylophora-pistillata-with-nutrient-driven-elevated-zooxanthellae-density-is-largely-explained-by-dic-limitation/.
Leave a comment